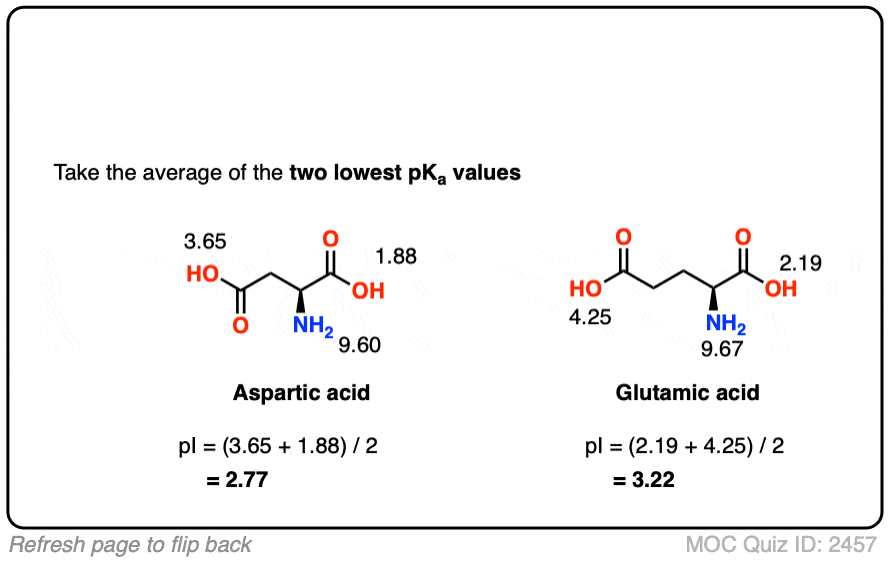
Isoelectric Point Of Amino Acids Definition . in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge.
from www.masterorganicchemistry.com
the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is.
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Organic Chemistry
Isoelectric Point Of Amino Acids Definition the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge.
From www.youtube.com
Amino Acid Isoelectric Point YouTube Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. the isoelectric point (pi) is the ph value at which the molecule carries no electrical. Isoelectric Point Of Amino Acids Definition.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Organic Chemistry Isoelectric Point Of Amino Acids Definition the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. the isoelectric point, pi, is the ph of an aqueous solution of an. Isoelectric Point Of Amino Acids Definition.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Organic Chemistry Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. the isoelectric point is defined as the ph at which. Isoelectric Point Of Amino Acids Definition.
From www.chegg.com
Solved The isoelectric point, pl, of two amino acids is Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the. Isoelectric Point Of Amino Acids Definition.
From www.slideserve.com
PPT Proteins PowerPoint Presentation, free download ID1428582 Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. discover the significance of the amino acid isoelectric point. Isoelectric Point Of Amino Acids Definition.
From www.youtube.com
B.2 Isoelectric point of amino acids (SL) YouTube Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid. Isoelectric Point Of Amino Acids Definition.
From www.youtube.com
How To Calculate The Isoelectric Point of Amino Acids and Zwitterions YouTube Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. the isoelectric point (pi) is the ph value at which. Isoelectric Point Of Amino Acids Definition.
From www.youtube.com
Isoelectric Point of Amino Acids I Part 20 I Biomolecules chemistry CBSE class 12 By Vani ma Isoelectric Point Of Amino Acids Definition the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,.. Isoelectric Point Of Amino Acids Definition.
From www.chemzipper.com
to Chem What is the Isoelectric points of amino acids? Isoelectric Point Of Amino Acids Definition in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. the isoelectric point (pi) is the ph value at which the molecule carries no. Isoelectric Point Of Amino Acids Definition.
From www.youtube.com
Isoelectric point of Amino Acid and Electrophoresis Technique. YouTube Isoelectric Point Of Amino Acids Definition in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while. Isoelectric Point Of Amino Acids Definition.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Organic Chemistry Isoelectric Point Of Amino Acids Definition the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and calculate the amino.. Isoelectric Point Of Amino Acids Definition.
From www.semanticscholar.org
Figure 1 from Isoelectric point prediction from the amino acid sequence of a protein Semantic Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. in between the two is an intermediate ph at which the. Isoelectric Point Of Amino Acids Definition.
From www.slideserve.com
PPT Amino acids PowerPoint Presentation, free download ID3960157 Isoelectric Point Of Amino Acids Definition in between the two is an intermediate ph at which the amino acid is exactly balanced between anionic and cationic forms,. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. the isoelectric point is defined as the ph at which no net migration takes. Isoelectric Point Of Amino Acids Definition.
From www.youtube.com
Isoelectric Point of Alpha Amino acids, Its definition and Utilisation to Separate amino acids Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. the isoelectric point (pi) is the ph value. Isoelectric Point Of Amino Acids Definition.
From chemistryguru.com.sg
Deduce Zwitterion and Isoelectric Point of Amino Acids Isoelectric Point Of Amino Acids Definition the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on average have no net charge. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. in between the two is an intermediate ph. Isoelectric Point Of Amino Acids Definition.
From www.doubtnut.com
What is isoelectric point amino acid ? How does it help in the sepration of amino acids Isoelectric Point Of Amino Acids Definition the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. discover the significance of the amino acid isoelectric point in biochemistry and learn how to detect and. Isoelectric Point Of Amino Acids Definition.
From chemistryguru.com.sg
Deduce Zwitterion and Isoelectric Point of Amino Acids Isoelectric Point Of Amino Acids Definition the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. the isoelectric point (pi) is the ph value at which the molecule carries no electrical charge. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide). Isoelectric Point Of Amino Acids Definition.
From glossary.periodni.com
Isoelectric Chemistry Dictionary & Glossary Isoelectric Point Of Amino Acids Definition the isoelectric point is defined as the ph at which no net migration takes place in an electric field, while the isoionic point is. the isoelectric point, pi, is the ph of an aqueous solution of an amino acid (or peptide) at which the molecules on. the isoelectric point, pi, is the ph of an aqueous solution. Isoelectric Point Of Amino Acids Definition.